lateral flow assay protocol
Each pairing between the test line antibody and conjugate is different and may require its own optimization. Although there are a number of different variations of the technology they all operate using the same basic.
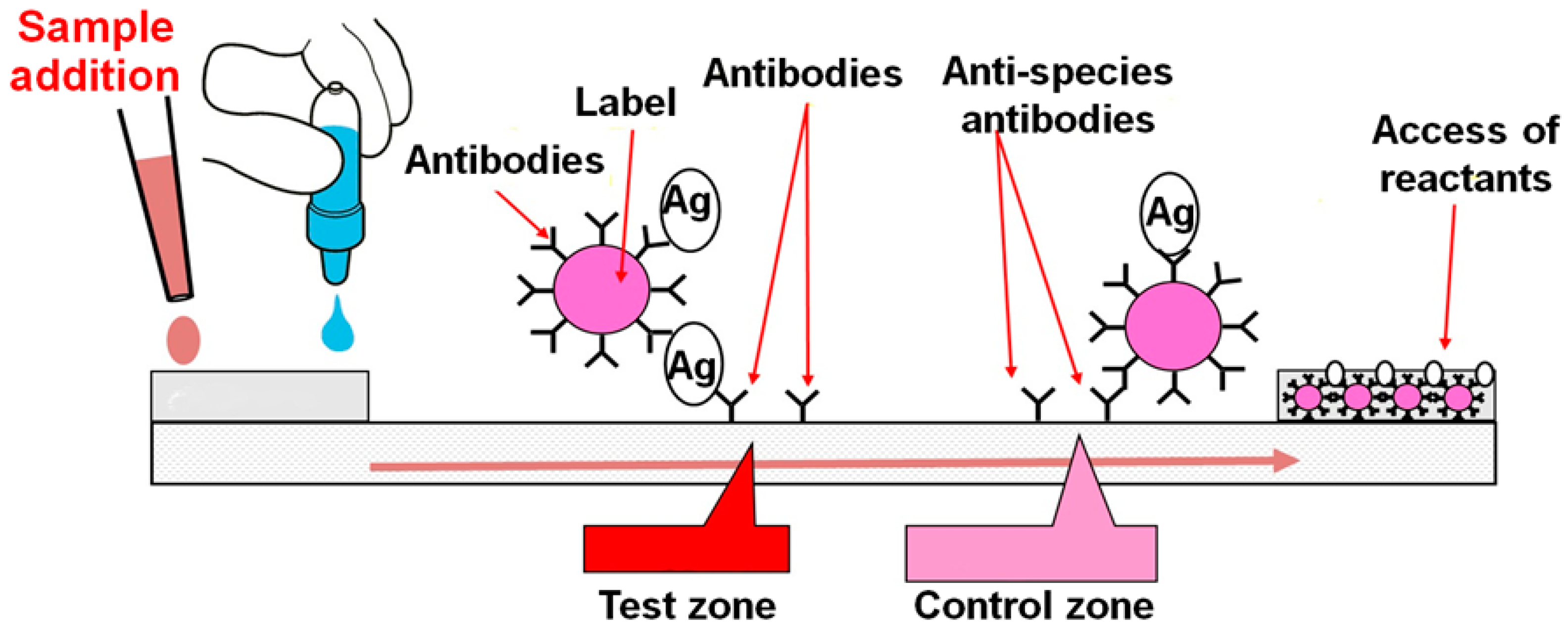
Biosensors Free Full Text Towards Lateral Flow Quantitative Assays Detection Approaches Html
This latest presentation on lateral flow immunoassay development will provide a general overview of lateral flow assays take you through the components of a.

. The first tests were made for the detection of human chorionic gonadotropin hCG. It combines universal LFA strips Lightning-Link and Gold conjugation technologies buffers and controls. Recent technology developments permit the use of inexpensive electronic readers for interrogating lateral flow strip test results thus avoiding the inevitable variation and subjectivity of visual inspection to assess the capture of reporter-labeled analyte on.
The lateral-flow assay is a rapid method to detect influenza virus. But the effectiveness of the technique in detecting flu viruses is unclear. Lateral flow assays LFAs are a widely used diagnostic tool for point-of-care diagnostics.
The simplicity and low cost of lateral-flow assays LFAs have made them one of the most used point-of-care PoC sensors 12 in various disciplines ranging from. The lateral flow assay LFA is a paper-based platform for the detection and quantification of analytes in complex mixtures where the sample is placed on a test device and the results are displayed within 530 min. Range than is needed from a qualitative assay.
Lateral flow test strips based on the principles of immunochromatography exist for a wide array of target analytes. If you have two pairs that are comparable in performance during the initial screening separate optimization may. NanoComposix has developed a variety of high-quality plasmonic nanoparticles including 40.
A successful diagnostic lateral flow assay is the product of many small optimizations that vary depending on the particle type target and system. Coronavirus Lateral Flow Assay LFA sample preparation protocol. Assay Procedure 7 6.
Lateral Flow Assay Optimization 10 7. LFAs require optimization of the component assembly sample pad conjugate pad nitrocellulose membrane NCM and absorbent pad on the plastic backing in preparation for a specific assay. Each diluted sample n60 will be tested on the lateral flow device LFD according to the manufacturers instructions.
Applications of lateral flow assay for ensuring the quality of food as reported in the literature have been discussed and some of the practical. Hence a meta-analysis would be performed to evaluate the accuracy of LFA in detecting influenza virus. Up to 10 cash back In this chapter we discuss various detection formats labels used for detection various components of lateral flow assay and biomolecules used for recognition used in lateral flow assay.
Lateral flow immunoassays Lateral flowimmunoassays also known as immunochromatographic assays or strip tests are immunoassays which have been designed to operate along a single axis. These assays have the benefit of being portable fast and relatively inexpensive. Up to 10 cash back The lateral flow assay offers a rapid and simple assay which allow early detection of viral infection in resource and equipment limited small laboratories.
LFTs are widely used in medical diagnostics in the home at the point of care and in the. Lateral flow assay LFA is a diagnostic procedure applied to detect and quantify certain analytes present in a complex mixture. A lateral flow test is an Assay also known as a lateral flow device lateral flow immunochromatographic assay or rapid test is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment.
Where LFDs require swab samples to be tested 200uL of sample will be. Universal Lateral Flow Assay Kit ab270537 is designed to enable simple and rapid development of proof-of-concept sandwich lateral flow immunoassays. Overview Universal Lateral Flow Assay Kit ab270537 is designed to enable the easy development of customized sandwich lateral flow assays by combining Lightning-Link and Gold conjugation technologies with an immunochromatography test performed on Universal-LFA.
Low development costs and ease of production of LFAs have resulted in the expansion of its applications to multiple fields in which rapid tests. Colloidal 40 nm gold nanoparticles are the industry standard for use as a probe in many lateral flow tests. General procedure for the card-based assembly of a lateral flow device consisting of conjugate preparation 1 striping of capture lines 2 spraying conjugate pad 3 assembly of cards 4 strip cutting 5 and the packaging into cassettes 6.
The kit includes everything needed for lateral flow assay development with any pair of capture and detection antibodies. This chapter describes the method for development of a lateral flow assay using gold nanoparticle probe for detection of viral antigen. The sample analyte which is to be detected is kept on a test device to display the results within a time period that ranges between 5 to 30 minutes.
Lateral flow immunoassays are qualitative POC tests that use antibodies to a protein of interest in any of a variety of bodily fluid samples including blood and saliva Carter et al 2020. The test samples required for the LFAs should mostly be in a. An important decision in the design of a lateral flow assay is the pairing of the test line antibody and the antibody or antigen conjugated to the reporter particle.
Lateral Flow Assay Development Guide. When a sample is added the sample will flow along the test device passing through the conjugate pad into the nitrocellulose membrane and then onto the absorbent pad. Determining necessary protocol parameters assembly and.
LFDs use immunoassay technology using nitrocellulose membrane coloured nanoparticles or labels and typically antibodies to produce results. Milwaukee Protocol version 6 updated November 2018 Protocol 1. I also read MERCKs guideline Rapid Lateral Flow Test Strips and it said that 005 SDS or 0005 Tritonx-100 should be added to the capture reagent solution to.
Today commercially available tests monitor ovulation detect infectious disease organisms analyze drugs of abuse and measure other analytes important to human physiology. Principles and characteristics of lateral flow strip assays are reviewed. The Premier Biotech COVID-19 IgGIgM Rapid Test Cassette is a lateral flow immunochromatographic assay for the detection of SARS-CoV-2 antibodies in venous whole blood serum or plasma.

Schematic Of A Lateral Flow Assay With Colloidal Gold As Label Download Scientific Diagram

Universal Lateral Flow Assay Kit Ab270537 Abcam
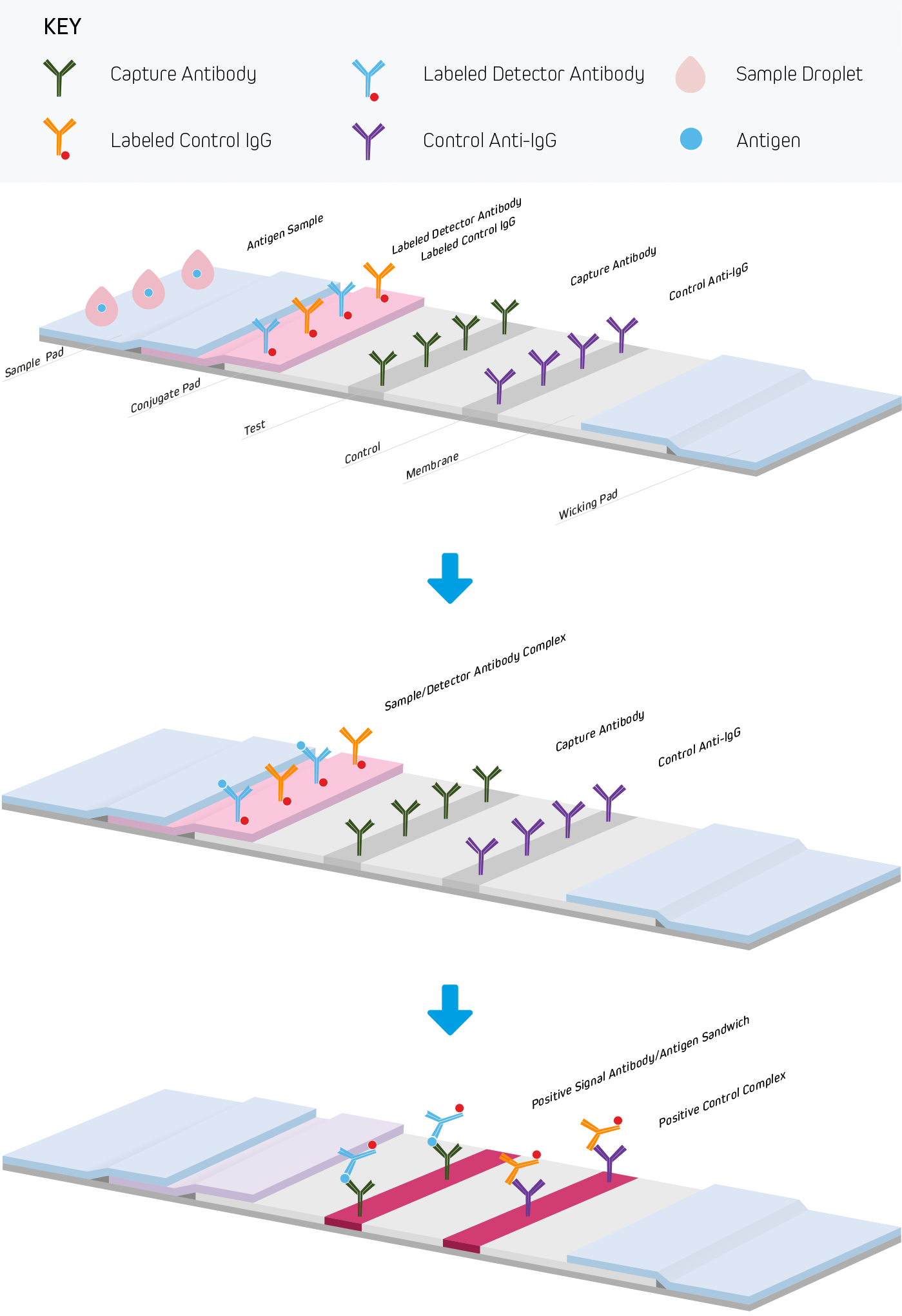
Lateral Flow Immunoassays Jackson Immunoresearch

Capillary Flow Control In Lateral Flow Assays Via Delaminating Timers
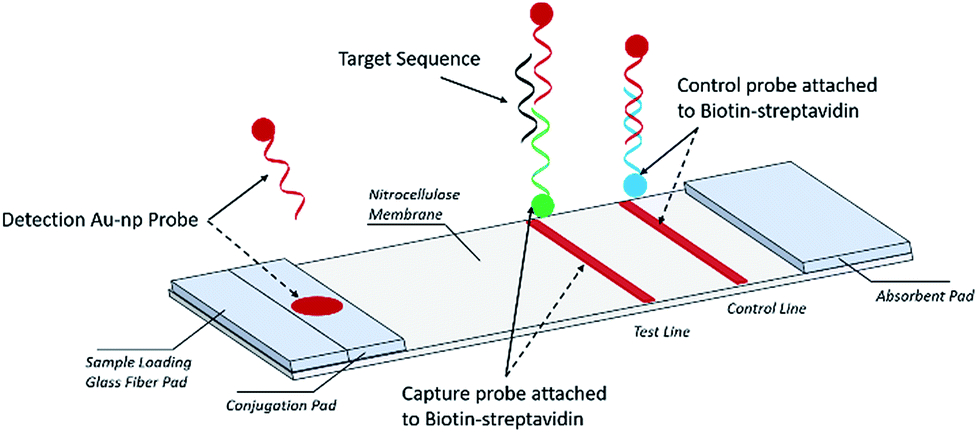
Simple Lateral Flow Assays For Microbial Detection In Stool Analytical Methods Rsc Publishing Doi 10 1039 C8ay01475b
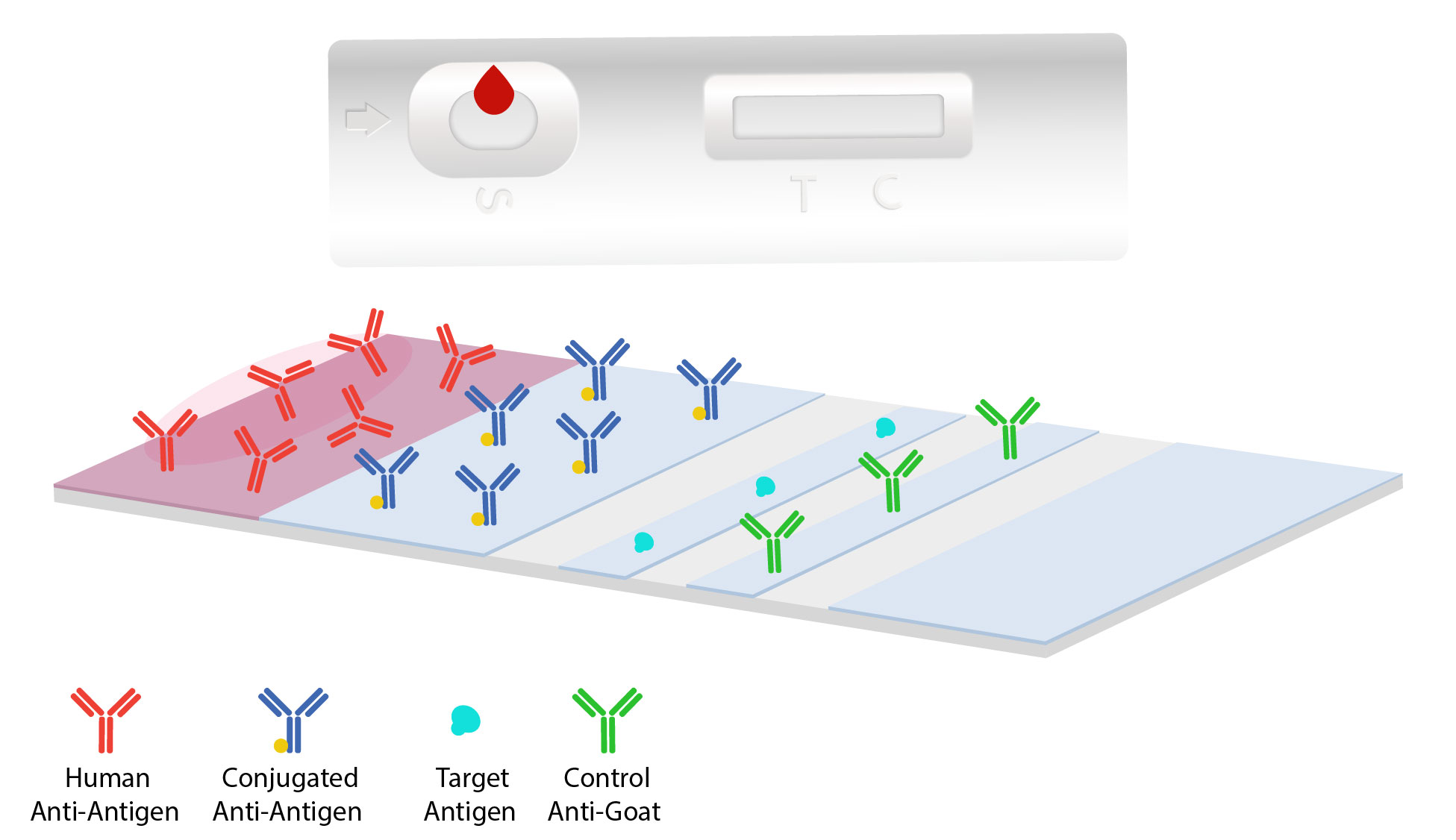
Lateral Flow Assay Development Leinco Technologies

Tutorial Design And Fabrication Of Nanoparticle Based Lateral Flow Immunoassays Nature Protocols

Lateral Flow Webinar A Guide To Lateral Flow Immunoassay Development Youtube

Steps In Lateral Flow Immunoassay Lfia Based Covid 19 Diagnosis A Download Scientific Diagram
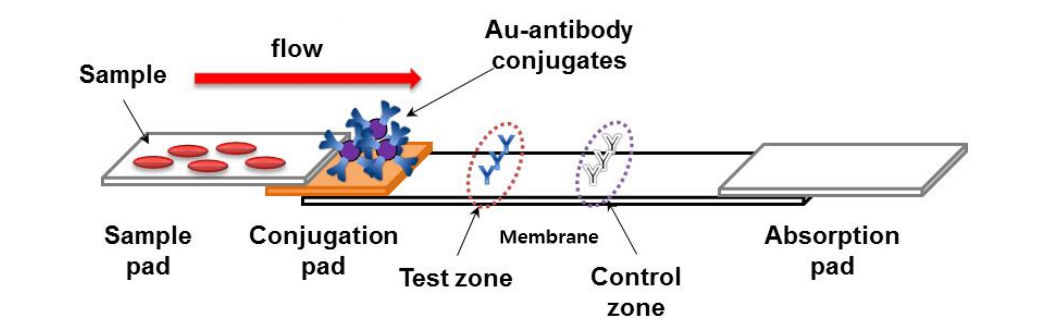
Lateral Flow Immunoassay Creative Diagnostics

Lateral Flow Test An Overview Sciencedirect Topics
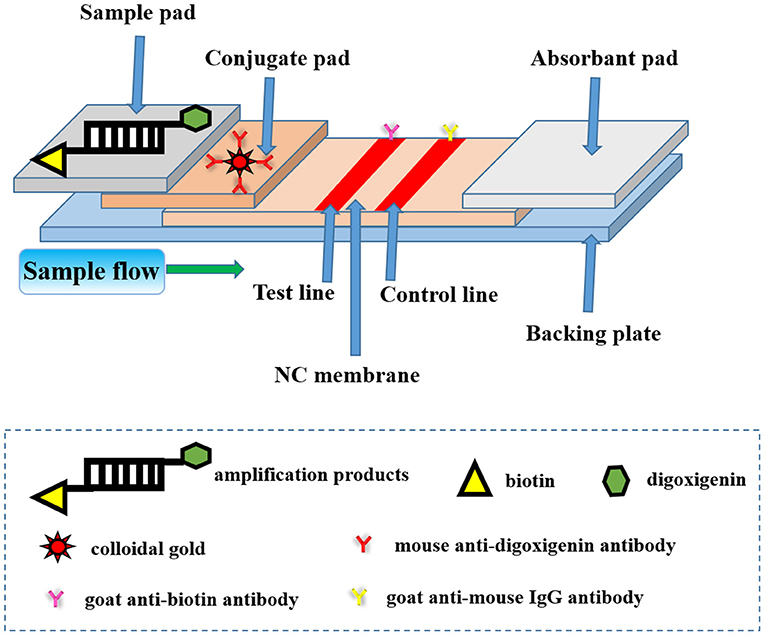
Frontiers A Novel Lateral Flow Assay For Rapid And Sensitive Nucleic Acid Detection Of Avibacterium Paragallinarum Veterinary Science

Lateral Flow Assay Schematic Design A A Lateral Flow Test Strip Download Scientific Diagram
Lateral Flow Rapid Test Assay Optimization Nanocomposix
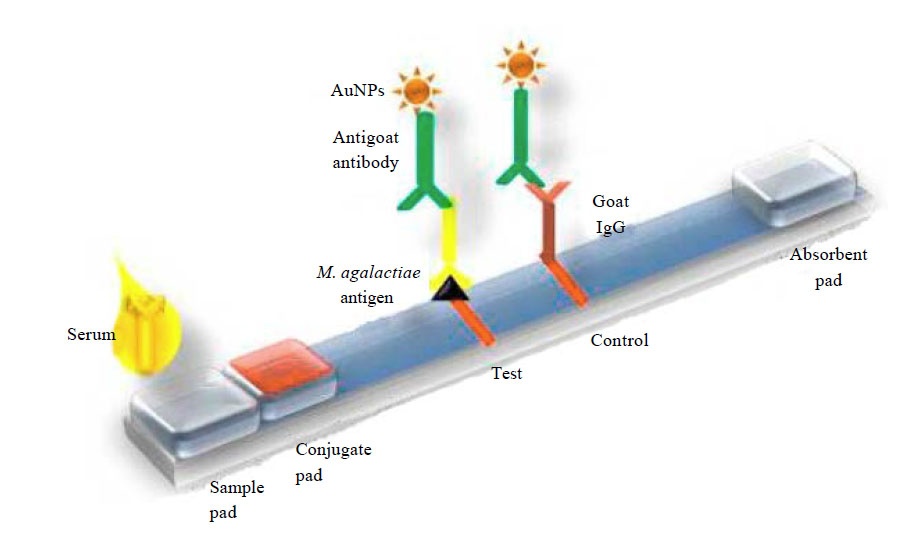
Molecular Diagnostics Lateral Flow Assay

Schematic Design Of A Lateral Flow Test According To Ref 68 A Download Scientific Diagram
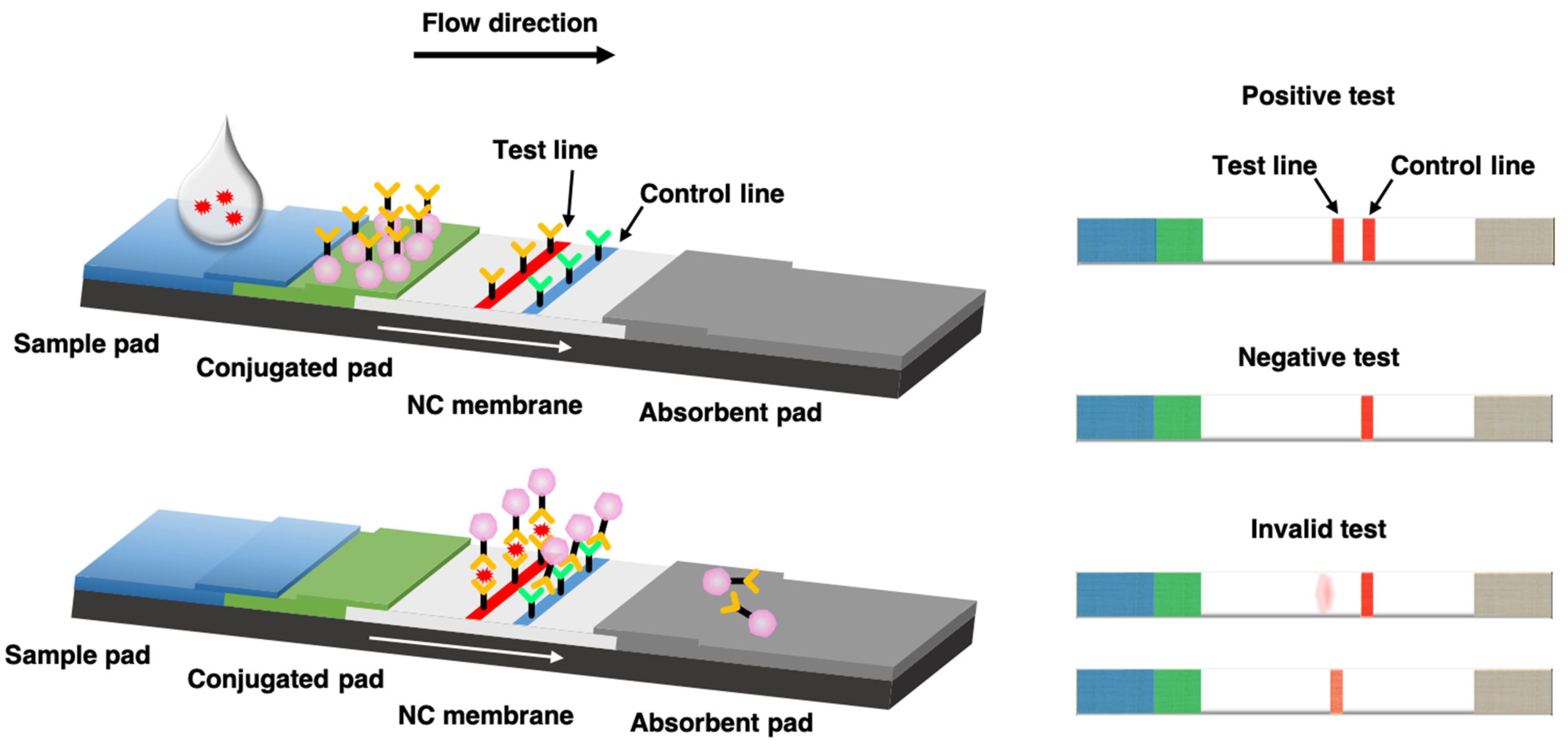
Biosensors Free Full Text Recent Advances In Novel Lateral Flow Technologies For Detection Of Covid 19 Html
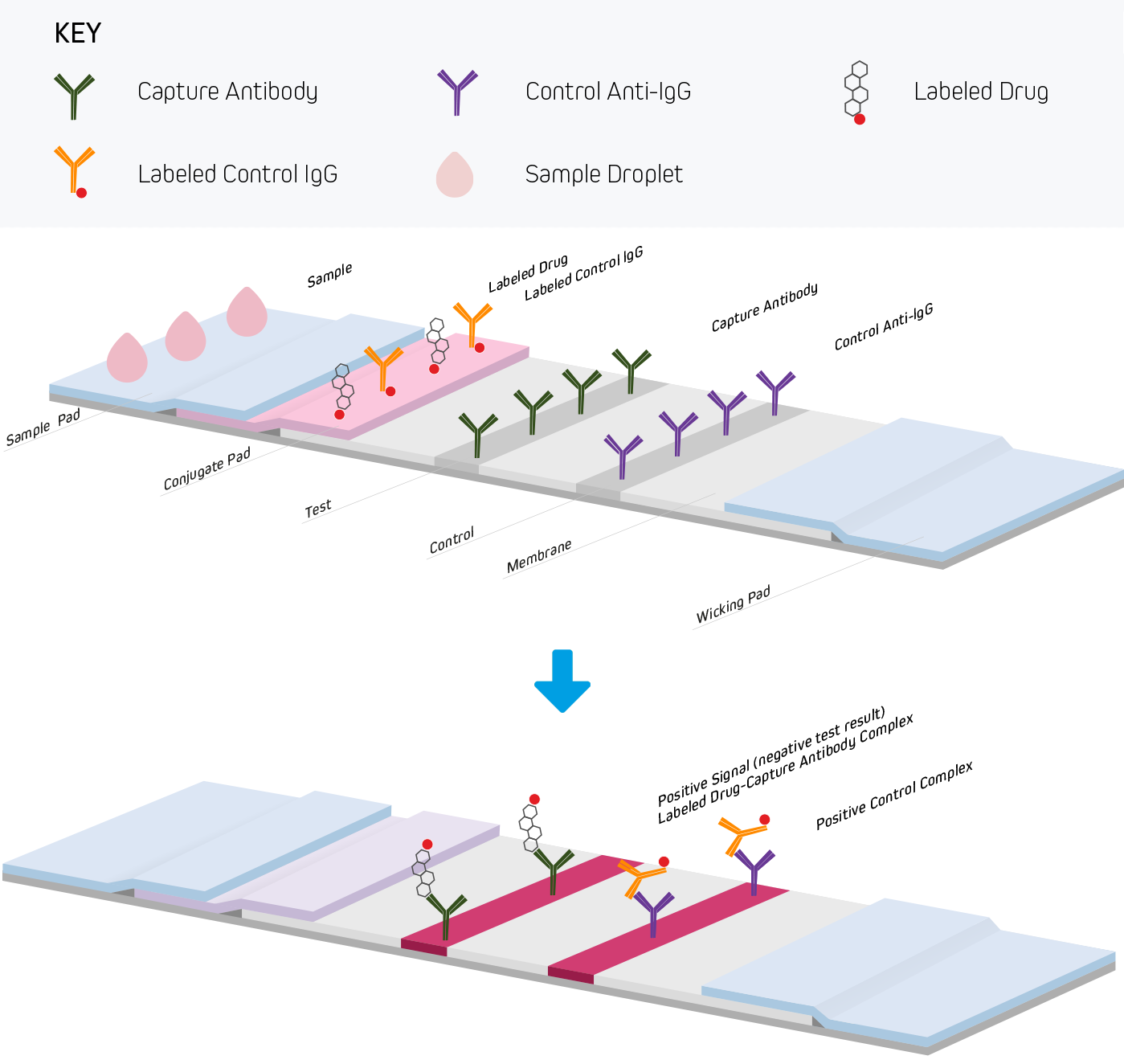
Lateral Flow Immunoassays Jackson Immunoresearch

Principle Of Competitive Lateral Flow Immunoassays The Device Is Download Scientific Diagram
Comments
Post a Comment